Mass production with additive manufacturing –
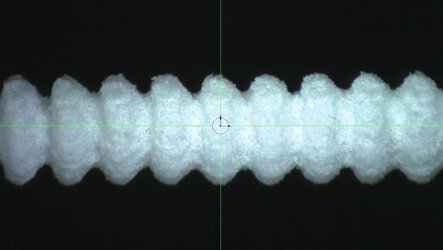
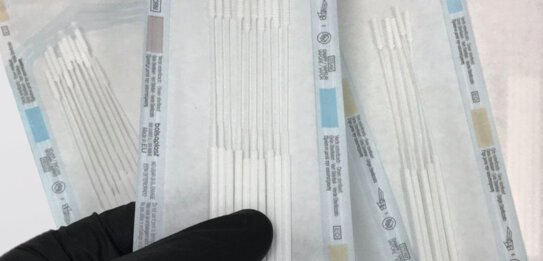
3D printed nasal swabs with porous-specific activated surface on the head plus highly flexible stick
Production of Medical Devices with Industrial 3D Printing in Europe and North America
The COVID-19 pandemic tested global health care capabilities. Supply chains were disrupted, requiring local agile solutions for certified medical devices. Many companies are collaborating with their local governments to provide critical medical devices such as ventilator components, ICU critical parts, face shields, face mask filters, etc., to fight the shortages.
In this context, a collaborative R&D consortium was created in March 2020 to develop certified medical devices and ramp up mass production in North America and Europe. This consortium included Aenium Engineering and Burloak Technologies, who worked closely with EOS’ Additive Minds applied engineering team, the Canadian and Spanish health authorities and hospitals, as well as a Spanish genomic laboratory (ITALCyL).
This is the first 3D-printed nasal swab certified by a government agency and was made possible by collaboration between Aenium, Burloak, Additive Minds and many others. It demonstrated the great opportunity for empowering private-public consortia in the fight against global challenges using industrial 3D printing – an inspiration for others worldwide.
Challenge
The demand for COVID-19 test kits combined with the empty inventory of conventionally manufactured nasal swabs made it clear that additive manufacturing would be the ideal technology for bridging this gap. However, there were challenges that needed to be overcome.
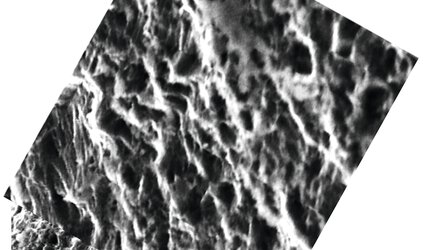
The first challenge was the sampling itself. It is essential for the test swab to preserve the virus’s integrity to allow RNA detection by means of reverse transcriptase real-time PCR. A solution is needed with a porous-specific activated surface on the head plus a highly flexible stick which cannot be manufactured conventionally.
The second challenge was the high demand for a cost-effective solution. Aenium and Burloak developed a model for producing up to 40 000 swabs per day on multiple EOS additive manufacturing systems.
And the third challenge: Nasal swabs are a class IIa medical device, requiring a specific certification for testing and qualification to fulfill medical standards. Therefore, a typically long certification procedure with the public administration and laboratories to approve the intended use was required.
Solution

Aenium started clinical trials with the Spanish authorities on April 25, 2020, and performed hundreds of clinical comparisons with commercial nasal swabs. In the clinical trials, RNA was processed by one-step RNA real-time PCR using two kits RT-PCR (BGI) and ref. RR064A (Takara) to detect SARS-CoV-2. Both nasal swabs included an endogenous control, obtaining positive results compared with the same clinical trials over other certified conventionally manufactured commercial swabs. Aenium and Burloak developed a RNA-free nasal swab with specific laboratory-tested parameters.
The nasal swabs were manufactured on EOSINT P 385, EOS P 396 and EOSINT P 760 systems using EOS PA2200 material.
Rapid Manufacturing Systems contributed to the mass production vision of offering market value for the nasal swabs industrialization. Based on this success, more products are in the pipeline (e.g. pediatric nasal swabs).
Results
Aenium is working with the Spanish government and hospitals to produce hundreds of thousands of medical nasal swabs for use in COVID-19 test kits. ITACyL, a public laboratory, and other Spanish laboratories have tested the performance and done the clinical validation to successfully achieve AEMPS (Spanish Agency of Medicines and Medical Products) certification for the mass production of this IIa class medical device.
Burloak is helping North American hospitals and public health systems to increase production capabilities and fight against stockouts of this critical medical device.
Want an offer or an appointment? We will be happy to advise you on the right additive manufacturing solution for your application
Thank you for your message.